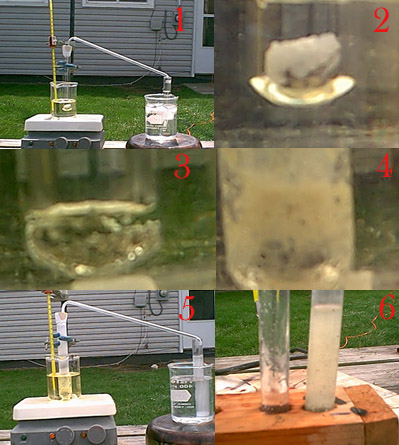
April 10, 2004

The principle here is that urea is reacted with nickel and by this method hydrazine and nickel carbonyl are formed, the nickel carbonyl decomposing to nickel and carbon monxoide (From patent US 2717201):
Ni + NH2CONH2 ---> Ni(CO)4 + NH2NH2
Ni(CO)4 ---> Ni + 4CO
Picture #1 shows the setup I used to try this reaction, an equal weight of nickel in the form of turnings and chunks along with urea are in the reaction test tube on the left of the picture. There is also a magnetic stir bar in there but nickel is ferromagnetic and it bogged down the stir bar. It is sitting in a oil bath, this test tube is plugged with a rubber stopper that is wrapped in teflon tape since I read in several places that hydrazine should not contact rubber. A glass tube ran from this tube to the next one which was filled with a cold dilute H2SO4 solution, the premise was that hydrazine formed would volatize and travel to the next test tube, where it would be precipitated in the form of the slightly soluble sulfate.
Picture #2 is just the reaction mixture prior to heating, and #3 is the initial molten sate at about 137C where it was held for 20 minutes, bubbles were coming over but urea decomposes at this temperature so it was indicative of nothing. The temperature was gradually increased over the course of an hour, around 200C picture #4 shows the urea decomposing quite readily but nothing had precipitated in the other test tube. As picture #5 shows some of the urea had sublimed into other parts of the tube making it opaque but there was no reaction visible except that the urea had turned a very slight shade of green so the nickel metal was being very slightly attacked in some way. Picture #6 shows the reaction test tube with some water added, it is cloudy from reaction byproducts, but I got no evidence of hydrazine.
I consider this reaction a failure, I used what I learned from this reaction in my second try on September 27, 2004.
April 18, 2004

This reaction was a simple test to see if the battery charger I had bought had enough power to produce sodium from molten sodium hydroxide. It had the capacity to charge at 12V and up to 15A. Sodium was placed onto a Pyrex watch glass and moistened with a small amount of water. Nickel electrodes were inserted into the pile of hydroxide pellets and electrolysis commenced. Only a small amount of current was carried by the mass but as the current went though the high resistance mass it heated, dissolved more NaOH and conducted more which caused it to heat, and dissolve more NaOH. Eventually by picture 3 I was at molten NaOH, all the water volatizing out during the early electrolysis and small globules of sodium were being produced, they are the shiny dots in pictures #2 and 3. Small microscopic particulates of NaOH flew off through the whole reaction and some went into my eyes, they burned badly so eye protection is a must, around picture 4 in the bottom left hand corner you can see little dots on the table where the molten NaOH spattered there.
NaOH dissolves glass, when I was done and cleaned off the watch glass there was a hole nearly a centimeter deep right at the point where the melt was the hottest, it very nearly ate its way though. Picture #4 shows just how hot the mixture got, scorching the table I was doing the experiment on. The electrolysis commenced best when the electrodes were near to one another and somewhat close to the surface, thus increasing the current density. NaOH fumes also rose up and created a problem, I had a sore throat for a few days.