|
Question I want some sodium fluoride, and have a quantity of
ammonium fluoride on hand. So, I'm planning on doing the obvious: My bookshelf has yielded only one relevant statement, that "NaF is slightly corrosive to glass". That would be referring to an aqueous solution, nothing else present, slightly alkaline by hydrolysis. Making it acid would make it very corrosive; maybe going the other way is protective. Do you think that making the solution strongly alkaline with an excess of NaOH is sufficient? Or do I need to precipitate the residual flouride with something like Ca++? Or maybe complex it with something? What would you do? Answer Sodium fluoride is going to be slightly corrosive to glass, though in your case it should be even less corrosive than usual due to the equilibrium: F-(aq) + H2O ---> HF(aq) + OH-(aq) That's where the bulk of the corrosivity comes from in terms of sodium fluoride eating glass, because hydrofluoric is a somewhat weak acid it's the equilibrium of the conjugate base (fluoride ion) converting back to the free acid. With your extra hydroxide in there from ammonium hydroxide you'll hopefully be driving the equilibrium far enough to the left to avoid some of those corrosivity issues (Which is exactly what you suggested). Honestly I like your plan, by avoiding acids you significantly reduce the risks of the procedure and damage to glassware. The main sticking point that I worry about is how the solubility of sodium fluoride is going to be affected by the pH of the solution. Here are the initial solubility's that we are looking at(1): Ammonium Fluoride: v.s. (Very Soluble) Not very useful save for the sodium fluoride solubility which you probably already knew. Because the solubility of sodium fluoride in a concentrated ammonia solution is probably not going to be readily available from any primary source I did some scrounging on the internet and found US patent 4113831. This patent covers the recovery of sodium fluoride and aluminum from spent carbon liners from aluminum electrolytic cells. The process described is the extraction of the carbon liners with strong caustic (sodium hydroxide) to give a solution containing sodium fluoride. The interesting bit is: "It is a further object of this invention to provide a system for the recovery of sodium fluoride from the caustic extract of spent cathode liner by the addition of ammonia or other solubility suppressant for sodium fluoride" Ammonia is mentioned several times throughout the patent as a solubility suppressant for sodium fluoride and is used to precipitate it. Although they saturate the solution with ammonia to get the maximum effect, it seems as if any amount would help, the patent also mentions: "Specific solvents showing solubility suppression effects when added to an aqueous solution saturated with sodium fluoride include: ethanol, mono-isopropanol amine, pyridine, morpholine, dimethylformamide, ethylenediamine, ethylene glycol, methanol, acetone, isopropanol and n-butyl alcohol."
So perhaps adding some ethanol or acetone after the precipitation is complete might help to drive out a few more percent yield of your sodium fluoride. Just a thought for consideration if your yield seems low. Regardless, here is how I would approach it with the knowledge at hand. Using the equation: NH4F + NaOH ---> NH4OH + NaF I would calculate out the stoichiometric amount of sodium hydroxide to react with a given amount of ammonium fluoride. This amount will be slightly off due to the way sodium hydroxide tends to pick up moisture (Commercial is approx 97% usually). I would dissolve my sodium hydroxide in a minimum amount of room temperature water then add 10% for good measure to reduce occlusion of impurities and my ammonium fluoride in a minimum amount of water plus 10% or so for the same reasons. With magnetic stirring or consistent stirring (to reduce areas of relative high concentration) I would add the the sodium hydroxide to the ammonium fluoride solution (if you go the other way with it, you will have a large excess of hydroxide to ammonia and would end up forcing more of your ammonia out of solution). After all of your hydroxide is added allow the mixture to set and settle then filter (gravity filtration would be best but could take a while, if using vacuum filtration watch for the ammonia causing a ruckus and boiling off). After you filter off your sodium fluoride you will be left with your solution of mostly ammonium hydroxide with some sodium fluoride dissolved in there. Hopefully the amount is very small (since it is depressed from its normal value) but to excise it all together, cool your solution down to 5ºC or so and add drop by drop a somewhat dilute solution of barium hydroxide. This will precipitate out your fluoride as barium fluoride, once additions stop precipitating fluoride allow to settle and filter. Solubility of barium fluoride is only 0.17 g / 100 ml at 10ºC(1) and hopefully the solubility will be further depressed by the ammonia solution. Once that is filtered off you should be left with a fairly pure NH4OH solution that you should be able to use directly or concentrate however you would like. I chose barium hydroxide simply because the hydroxide is more soluble so you will be less likely to just add to your concentrated ammonium hydroxide solution and get precipitation of a hydroxide (with a calcium hydroxide solution the calcium hydroxide is somewhat more insoluble so adding it to a concentrated hydroxide solution might get it to precipitate without precipitating out the fluoride.) You could probably use calcium hydroxide but you would have to be aware of this and watch out for this occurring. If you want to go in a somewhat more radically different direction, you could do this reaction almost neat. Grind up your sodium hydroxide, make sure your ammonium fluoride is finely powdered, add a small amount of water to make your hydroxide into a paste and add in your ammonium fluoride bit by bit. Overall reaction is going to be the same, but your ammonia will be produced directly as a gas, set up some traps in line and absorb it. Concentrated sodium hydroxide is widely touted as being bad for glassware, it will eat it, but it takes lots of time or lots of heat, there should be no worries here. The only difficulty is that the reaction would produce its own water and dissolve everything so your gas evolution would stop and you would have to heat to drive it along. Also I don't think this method is as clean and might get messy. But still it is something else for you to consider. I would also recommend you do any of these reactions on the small scale first (just to make sure the precipitations are spontaneous and don't easily go to supersaturation) and also take note of the toxicity of fluorides in general. Hopefully this gives you enough to work with and decide your course of action. Best of Luck, -Chemist (1) Hodgman, Charles D. Handbook of Chemistry and Physics. 39th ed. Cleveland, OH: Chemical Rubber Publishing Co. 1957 (2) Orth Jr., George Otto, Orth, Richard D. US Patent 4,113,831, 1978. On the question of corrosiveness, you seem to have left me
where I started: high pH is probably enough to keep the HF concentration down to
a tolerable level, but to be sure, I'd better precipitate as much of the F- as
possible. That's the one-line answer, right? It's amazing how these seemingly
trivial reactions get complicated as soon as one looks a little closer. I had
been wondering what effect the NH4OH would have on the solubility of the NaF.
That kind of question is usually easier to answer in the lab than the library.
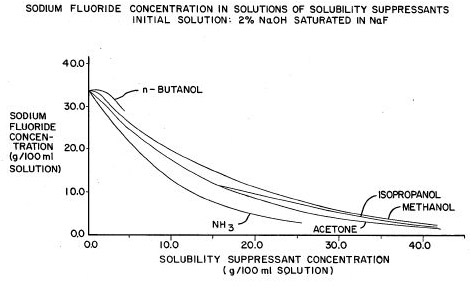
Answer Nice catch on the scale of the graph. Ironically enough (and you might have experience with it) patents have a tendency to have numerous inaccuracies despite the time and preparation put forth into preparing them. I think the mistake there does not lie in gross incompetence though but in either a missing decimal point or the solubility should have been expressed in g/L as it is in the rest of the patent (see capture from patent below).
Note that the above quote says that the solubility is 41 g / L, not ml so that's fairly close to what values we've seen elsewhere. I also re-cropped the image that is included in the first response from me to include the title of the graph which notes that it is a saturated solution of NaF in 2% NaOH which likely decreased the initial solubility (though I would not have thought to that extent). Anyway, the graph that is included in that patent is wrong, either in the scale or the labeling of the scale. And I agree, if you are going to recover the ammonia then adding a volatile solvent isn't going to help matters. As for my preference for barium hydroxide, it is just that barium hydroxide is so much more soluble, with calcium hydroxide you also get precipitation of calcium carbonate from standing of the solution, it just gets a little messier (unless you are a tidy chemist and prepare solutions fresh, then it's easier). Interesting mentioning the colloid difficulties that could occur, I've heard of precipitations of calcium salts coming out as messy gels, but dropping out such a small amount of fluoride that might be lingering, it didn't seem like that would be much of a problem. The bit I mentioned about doing things almost neat was more me speculating than anything, it would make things much more complicated, but it was something interesting to consider. -Chemist |